Abstract
Monocytes are important participants in various pathological conditions including inflammation, autoimmunity, atherosclerosis, metabolic disorders and cancer. Plasticity is a fundamental attribute of monocyte cell population; hence it is possible to differentiate those cells into the macrophages associated with the tumor. The study that shows how monocyte and macrophage cells effect tumor and stromal cells can pave the way for innovative methods of treating a wide range of diseases. The aim of this work was to investigate the effect of CD14+monocytes on mesenchymal stem cells and HeLa cells in double co-cultures.
Materials and methods: in order to create a double co-culture, we used adipose-derived mesenchymal stem cells (ADSCs), HeLa cells and CD14+ monocytes which were derived from fresh peripheral blood of a healthy donor by using EasySep™ Human CD14 Positive Selection Kit (StemCell Technologies Inc). Before creation of the co-culture, all cells were stained with vital membrane fluorescent dyes: Vybrant Cell-Labeling Solutions DiD (red) for ADSCs, DiI (yellow) for HeLa and DiO (green) for CD14+ monocytes cell membranes were labeled according to the manufacturer's instructions (Thermo Fisher Scientific, USA). After 72 hours of cultivation the cells were analyzed and sorted depending on the fluorescent spectrum using FACS Aria III (BD Biosciences, USA). After sorting separated populations were exposed to test for viability (MTS-test).
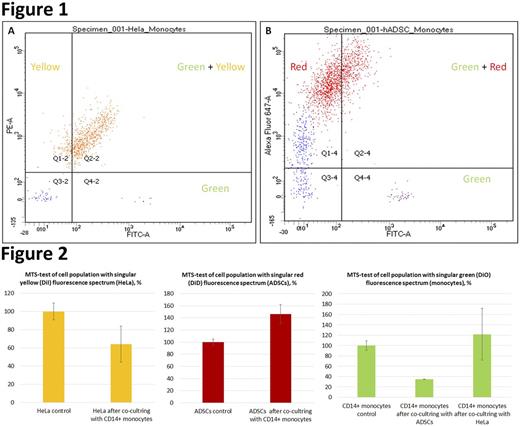
Results: the result of the co-cultivation of CD14+ monocytes (green) with HeLa (yellow) and CD14+monocytes (green) with ADSCs (red) was the high attraction of monocytes to HeLa and low attraction to stromal cells. Flow cytometry data after 72 hours of co-cultivation showed the active exchange of membrane components, up to the complete disappearance of monocytes during co-culture. This phenomenon was observed in co-culture with tumor cells (38.5% mixed fluorescence spectrum cell population) as well as in co-culture with adipose stromal cells (25.3% mixed fluorescence cell population) (Fig. 1). MTS-test showed a significant decrease in the viability of HeLa cells after co-cultivation with monocytes (36% compared to control), ADSCs demonstrated viability increase (46% compared to control), monocytes after co-cultivation demonstrated a decrease in viability after co-cultivation with ADSCs (65% compared to control) and insignificant increase in viability after co-cultivation with HeLa cells (Fig. 2).
The results obtained for monocytes can be explained by the specificity of the population choice in the process of sorting the co-cultures. As mentioned above, for the MTS test we selected those populations that had only one fluorescence spectrum. Monocytes which possessed the single fluorescence spectrum probably did not have direct interaction with other cells in the co-culture. This can be explained by the fact that these monocyte populations did not have time to react with other cells, or because these populations had properties different from other populations of monocytes in co-cultures. This study was supported by RFBR grant 16-34-60201.
Fig. 1. Flow cytometry assay after 72 h of co-cultivation of CD14+monocytes and HeLa cells (A) and CD14+monocytes and ADSCs (B).
Fig. 2. MTS-test assay of populations with the singular fluorescence spectrum after 72 h of co-cultivation of CD14+monocytes, HeLa cells and ADSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal